Scientific Calendar October 2024
Monitoring DAPT using LTA
How can you utilise LTA (light transmission aggregometry) to monitor DAPT (dual antiplatelet therapy) effectively?
Determining maximum platelet aggregation in % from a single arachidonic acid agonist concentration
Determining the PT in INR
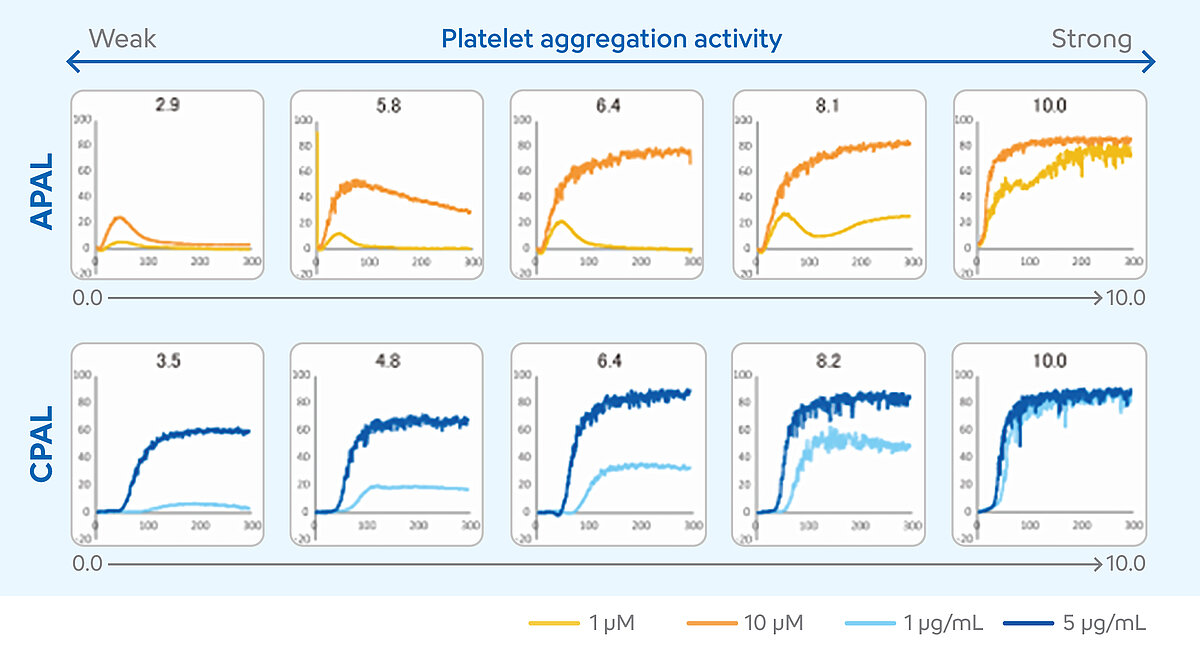
Determining the APAL and CPAL score
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
Nowadays, many tools are available that aid in assessing the platelet function. The most established and well-known method is light transmission aggregometry (LTA) according to Born. This measurement method was developed in 1963 and is the gold standard for investigations on inherited platelet function disorders. [1, 2] The measurement is based on a change in light transmission of the platelet-rich plasma due to platelet activation and aggregation after the addition of a platelet agonist. [1] Although limited by the guidelines to research purposes only, the LTA can also be used to monitor antiplatelet therapy. [3]
Dual antiplatelet therapy with clopidogrel or another P2Y12-inhibitor and aspirin has proven to be an established treatment strategy recommended by guidelines for patients suffering from cardiovascular disease. The pharmacological effect of antiplatelet drugs is known to vary among individuals. It has been reported that many individuals do not respond to clopidogrel due to CYP2C19 gene polymorphisms. [4–6] The antiplatelet effect of aspirin is reduced in patients with so-called ‘aspirin resistance’ due to single nucleotide polymorphisms (SNP) affecting COX-1 and platelet function, inflammation and metabolic syndrome. [7–9]
In recent years, LTA became more standardised thanks to fully automated coagulation analysers of the CS-Series (excl. CS-1600) and CN-Series (all Sysmex Corporation, Kobe, Japan) which reduce the manual intervention by the operator, significantly improving precision and repeatability of the results.
Table 1 LTA using a semi-automated analyser vs. CS-and CN-Series analysers
| Semi-automated | Operation steps | CN- / CS-Series |
| Manual | Blood sample collection and preparation | Manual |
| Manual | Preparation of stir cuvette | Minimised |
| Manual | Sample dispensing | Automated |
| Manual | Agonist dilution | Automated* |
| Manual | Agonist dispensing | Automated |
| Automated | Aggregation detection | Automated |
| Automated | Result output | Automated |
*CN-Series only
The interpretation of LTA measurement results requires a high degree of expertise. Various numerical results and the curve progression must be included in the evaluation of individual cases. In recent years, these conditions have limited the use of LTA in the monitoring of dual antiplatelet therapy (DAPT). To solve this problem and facilitate the interpretation of the results, the two-agonist concentration method was developed for the CS- and CN-Series analysers. [10] In this method, patient samples are measured with two concentrations of an aspirin- and a P2Y12-inhibitor-sensitive agonist. The results from the two concentrations are translated into a score, the platelet aggregation level (PAL). This score can be used to estimate whether the DAPT is sufficiently effective or whether the patient may require a therapy adjustment. The PAL is an additional function of the analyser and uses the platelet agonists ADP as a P2Y12-inhibitor-sensitive agonist and collagen as an aspirin-sensitive agonist. The ADP-induced PAL (APAL) is calculated from the measurement results in a concentration of 1 µM and 10 µM, and the collagen-induced PAL (CPAL) from the measurement results in a concentration of 1 µg/mL and 5 µg/mL. Both APAL and CPAL are calculated using the area under the curve (AUC). [11-13]
The scores may range from 0 to 10. Higher PAL score values indicate higher levels of platelet aggregation, suggesting a poor or absent response to DAPT, while lower PAL score values indicate lower levels of platelet aggregation, suggesting an appropriate response to DAPT. [14]
Studies on the PAL scores on CS- and CN-Series analysers unveiled imprecision of below 5% in samples spiked either with aspirin (CPAL) or the P2Y12-inhibitor cangrelor (APAL) on a CN-Series analyser, and below 10% for cangrelor-spiked (APAL) and below 5% on aspirin-spiked (CPAL) samples on a CS-5100 analyser. PAL results correlate well between both analyser series as shown in table 2. [14, 15]
Table 2 Correlation between CN-6000 and CS-5100
| AGONIST | CONCENTRATION | N | R-COEFFICIENT | |
| ADP | 1 µM | 85 | 0.988 | y = 1.00x + 3.85 |
| 10 µM | 85 | 0.955 | y = 0.89x + 11.44 | |
| APAL score | 85 | 0.971 | y = 0.91x + 0.94 | |
| COLLAGEN | 1 µg/mL | 82 | 0.996 | y = 1.02x - 0.83 |
| 5 µg/mL | 82 | 0.972 | y = 0.99x + 0.66 | |
| CPAL score | 82 | 0.994 | y = 1.00x - 0.08 |
Other studies have demonstrated an improved sensitivity of the PAL system compared to the conventional use of LTA without a score system. It was found that the PAL score reduced the variability in the same subject and proved to be more reliable for measuring the efficacy of antiplatelet drugs than maximum platelet aggregation in % in a single agonist concentration. [16]
Further research is currently underway to establish cut-off values for monitoring antiplatelet therapy using the PAL score that will ideally significantly reduce the recurrence of thrombotic events during treatment.
References
[1] Born GV. (1962): Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 194: 927–929.
[2] Kang J, Park KW, Palmerini T, et al. (2019): Racial Differences in Ischaemia/Bleeding Risk Trade-Off during Anti-Platelet Therapy: Individual Patient Level Landmark Meta-Analysis from Seven RCTs. Thromb Haemost. 119 (1): 149–162.
[3] Cattaneo M, Cerletti C, Harrison P, Hayward CPM, Kenny D, Nugent D, Nurden P, Rao AK, Schmaier AH, Watson SP, Lussana F, Pugliano MT, Michelson AD. (2013): Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J Thromb Haemost; 11:1183–1189.
[4] Cattaneo M. (2010): New P2Y(12) inhibitors. Circulation. 121 (1): 171–179.
[5] Bonello L, Tantry US, Marcucci R, et al. (2010): Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 56 (12): 919–933.
[6] Tantry US, Bonello L, Aradi D, et al. (2013): Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 62 (24): 2261–2273.
[7] Colantonio LD, Gamboa CM, Kleindorfer DO, et al. (2016): Stroke symptoms and risk for incident coronary heart disease in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Int J Cardiol. 220: 122–128.
[8] Cattaneo M. (2007): Resistance to antiplatelet drugs: molecular mechanisms and laboratory detection. J Thromb Haemost. 5, Suppl 1: 230–237.
[9] Cattaneo M. (2004): Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol. 24 (11): 1980–1987.
[10] Matsuo T, Ohki Y. (1977): Classification of platelet aggregation patterns with two ADP solutions (the double-ADP method) and its clinical application to diabetes mellitus. Thromb Res. 11 (4): 453– 461.
[11] Sakayori T, Watanabe Y, Kitano K, et al. (2019): Evaluating the Utility of a Novel Research Use Index in Platelet Aggregation Analysis Featured in an Automated Blood Coagulation Analyzer to Confirm the Effect of Antiplatelet Drugs. Sysmex J Int. 2019; 29 (1): 39–47.
[12] Omori Y, Ishida H, Sakayori T, et al. (2019): Evaluation of Fully Automated Blood Coagulation Analyzer Equipped with a Novel Analysis Method – Antiplatelet Responsive Index –. Rinsho Byori. 67 (3): 205–211.
[13] Sadakata T, Sakayori T, Watanabe Y, et al. (2018): Basic Evaluation of PAL Which is a Research Use Index for Confirming the Effect of Antiplatelet Drugs Installed in the CS Series and Comparison Study with the Index Installed in Existing Instrument. Sysmex J. 2018; 19 (2): 1–10.
[14] Sakayori T, et al. (2024): Analytical Evaluation of Platelet Aggregation Level on a Fully Automated Coagulation Analyzer CN-6000, and a Case Study of an Initial Absorbance of Platelet-rich Plasma. Sysmex Journal International Volume 34 No.1. Published 12 June 2024.
[15] Shimizu M, et al. (2019): Evaluation of a new analysis index of platelet aggregation test using CS-5100 with G-Type on a PRP313M. Japanese Journal of Medical Technology, Volume 68, Issue 3, Pages 501–506.
[16] Lecchi A, Capecchi M, Padovan L, Artoni A, Arai N, Shinohara S, La Marca S, Peyvandi F. (2024): Evaluation of an automated platelet aggregation method for detection of congenital or acquired platelet function defects. Blood Transfus. 22(4):350–359.