Scientific Calendar December 2024
Acute myeloid leukaemia (AML)
What are the characteristics of blasts in the WPC channel after the treatment with reagents?
Blasts are not strongly permeated by the lysis reagent and therefore show low fluorescence signals and high signals for cell size.
Blasts are strongly lysed, causing higher fluorescence signals and weaker size signals.
Blasts shrink during the reagent treatment, producing low fluorescence signals and signals of smaller size.
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
Acute myeloid leukaemia
Acute myeloid leukaemia (AML) is the most common type of acute leukaemia in adults and is characterised by the clonal proliferation of myeloid precursor cells within the bone marrow [1]. The highest incidence of AML is observed in Europe, particularly in the UK, showing an incidence of 4.05 per 100,000 people. However, AML is considered a rare disease and affects mainly the elderly [1]. In 97% of AML cases, genetic alterations arise in the stem cell precursors of the myeloid lineage, resulting in neoplastic changes and clonal proliferation [2]. These mutations are heterogenous and present a variety of cytogenetic and molecular abnormalities across different functional classes [3]. The WHO defined a cut-off for AML diagnosis that requires having at least 20% myeloid blasts in peripheral blood or bone marrow [4]. Diagnosing and monitoring AML necessitates a comprehensive approach consisting of haematologic and morphologic analyses, clinical flow cytometry, and molecular testing.
Detection of blasts with Sysmex WDF and WPC channels
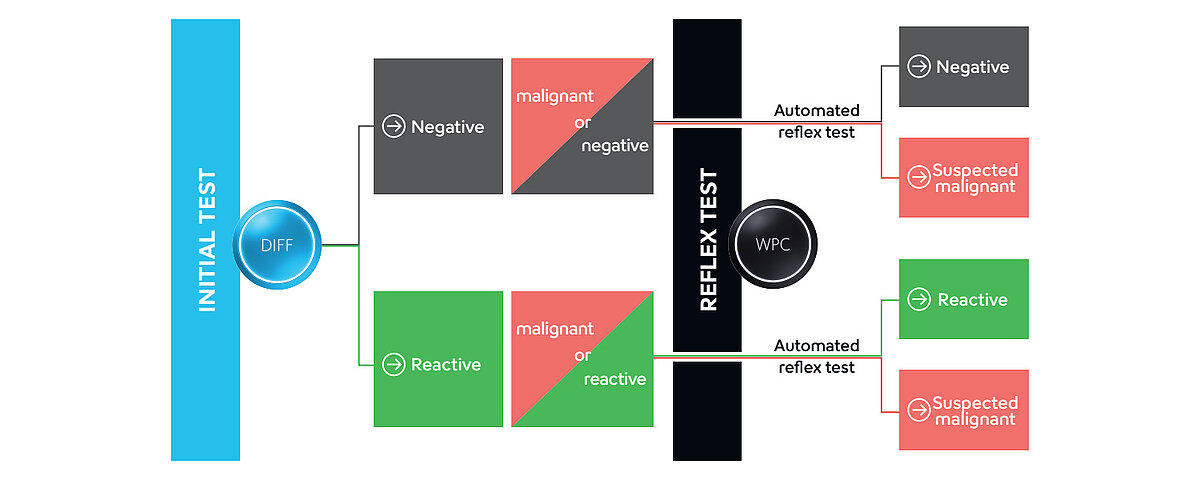
The classical CBC+DIFF analysis on Sysmex routine haematology analysers provides an initial indication for the presence of abnormal cells. This can be complemented by additional information gathered from the ‘white precursor and pathological cell’ (WPC) channel.
The membranes of white blood cells (WBC) are differently composed, depending on their maturity, function and activation status. The white blood cell differential (WDF) channel utilises a unique reagent combination that can separate WBC subtypes according to these differences in membrane composition and cytoplasmic content. The lysing reagent reacts rather gently, perforating the cell membrane and leaving the internal structure of the cells largely intact. This allows the fluorescence marker to enter the cell and label primarily RNA. Depending on the measurement signals of the cell clusters, i.e. fluorescence, forward- and side-scatter signals, specific flags are triggered, pre-classifying the sample as negative, reactive or potentially malignant. Based on this pre-classification in the WDF channel, the flag ‘Blasts/Abn Lympho?’ or the combination ‘Blasts/Abn Lympho?’ and ‘Atypical Lympho?’ can be triggered, leading to an automated reflex measurement in the WPC channel (Fig. 1).
In comparison to the WDF channel, the lysis reagent of the WPC channel has a stronger impact on membrane lipids, resulting in a higher cell membrane permeability. With the polymethine concentration of the WPC fluorescence reagent being higher than that of the WDF channel, it labels DNA inside the nucleus instead of cytoplasmic RNA. The special characteristics of different WBC can be used for subpopulation differentiation, e.g. between abnormal lymphocytes and blast cells (Fig. 2).
AML is characterised by the presence of blast cells from the myeloid lineage. Blast cells present a low lipid composition, which makes them more resistant to cell lysis even by the strong WPC lysis reagent. The cell permeability is reduced and results in a lower fluorescence signal combined with a high signal for cell size since the cells remain mostly intact. These characteristics allow a reliable identification of blast cells in the WPC channel.
Samples flagged during WDF measurement undergo reflex analysis and the abnormality is then either further classified by a more specific flag (e.g. ‘Blast?’ or ‘Abn Lympho?’) or the flag is removed altogether.
Samples that are suspicious for haematologic malignancies such as AML will undergo further specialised analysis. By flow cytometric immunophenotyping, suspicious cells are characterised in more detail by utilising these cells’ specific surface and intracellular markers. In the molecular lab, specific mutations can be evaluated by cytogenetic techniques such as fluorescence in situ hybridisation (FISH) and next-generation sequencing (NGS).
Including these disciplines allows clinicians to combine data from various tests, leading to a comprehensive understanding of the patient’s condition and the identification of an individually targeted and effective treatment strategy.
Case results
A 70-year-old female patient was undergoing several courses of chemotherapy and radiation for non-small cell lung carcinoma. She presented at an outpatient clinic after developing malaise and markedly decreased exertion. Blood testing on an XR-Series analyser initially revealed anaemia, thrombocytopenia, and slight leucocytosis (Fig. 3).
The flag ‘IG Present’ was triggered in the WDF channel. The immature granulocyte (IG) count includes promyelocytes, myelocytes and metamyelocytes and is relevant especially to patients who are highly susceptible to infections because of a suppressed immune system, e.g. due to chemotherapy. The presence of immature granulocytes indicates the severity of the early innate immune response.
In addition, the analyser triggered the flag ‘Blasts?’ from the WPC channel and the respective scattergram showed an increased presence of cells in the blast area (Fig. 4). This finding is indicative of the presence of acute malignancies, such as myeloid or lymphoblastic leukaemia.
Subsequently, a smear review was performed to inspect the morphology and count of the cells, revealing that 75% were blast cells. The subsequent immunophenotyping in the clinical flow laboratory confirmed the suspicion of AML.
References
[1] Dong Y et al. (2020): Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol; 9:14.
[2] Pelcovits A et al. (2020): R I Med J; 103(3). Acute Myeloid Leukemia: A Review.
[3] Kayser S et al. (2023): The clinical impact of the molecular landscape of acute myeloid leukemia. Haematologica; 108(2):308–320.
[4] Khoury JD et al. (2022): The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia; 36(7), 1703–1719.